Why Trans Fats Do Harm |
 Hebrew |
Many manufactured foods contain partially hydrogenated vegetable oils. Since the products of partial hydrogenation are generally solids at room temperature, these products may be classified as fats, and since a large percentage of the unsaturated bonds that remain in them take on a trans configuration during partial hydrogenation, they are commonly called trans fats. The term trans fatty acids is often used in connection with trans fats, because the trans unsaturated bonds in them are located in their fatty acid component.
Food manufacturers add trans fats to their products because they give foods a desirable taste and texture and dramatically extend their shelf life. Trans fats are also less expensive than the alternatives and do not become rancid. In addition, just as trans fatty acids form under the conditions of partial hydrogenation, they also form to some extent whenever vegetable oil is heated, and this extent becomes significant when the oil is heated at high temperatures for a long time, as in the case of commercial deep-fat frying. Trans fats are found in baked goods, including breads, pastries, pie crusts, biscuits, cookies, crackers, and frozen dough, as well as in deep-fry foods, such as French fries and doughnuts, in soup powders, in margarine, and in other vegetable shortenings.
There are growing concerns that trans fats may be harmful to our health. These concerns are based on reports that trans fats increase the risk of coronary heart disease and may cause obesity, metabolic disorders, and diabetes. Studies have shown that trans fats may raise our blood LDL (low-density lipoproteins or bad cholesterol) and lower our HDL (high-density lipoproteins or good cholesterol). For a quick summary of the mounting evidence underlying these concerns, see Trans Fats and Cardiovascular Health on the Harvard School of Public Health website.
In response to these concerns, many people are trying to avoid eating trans fats and would like to see them banned. On the other hand, many other people are finding it hard to accept that a seemingly minor change in the configuration of unsaturated chemical bonds can have such disastrous effects. The following section is intended to give you a good understanding of the physical meaning of the word trans with the hope that this understanding will help you make informed decisions regarding your health.
The Trans Configuration
Scientists use two words to describe the arrangement, or configuration, of the four atoms or chains of atoms that are attached to the carbon atoms joined by an unsaturated bond in a molecule of an organic compound when two of these atoms or chains of atoms are identical, as in the case of an unsaturated bond in a fatty acid, in which one hydrogen atom is attached to each of the carbon atoms joined by the unsaturated bond. One of these words is cis, which comes from a Latin word that means “on the same side.” The other is trans, which comes from a Latin word that means “across.”
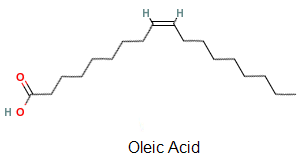
For example, the most abundant fatty acid in natural vegetable oils is oleic acid, which contains one unsaturated carbon–carbon bond with a cis configuration, as can be seen in the stick diagram of its chemical structure in Oleic Acid — Compound Summary. In this diagram the unsaturated bond, which is also called a double bond, is represented by the two parallel lines located midway along the molecule ( ), each single straight line represents a saturated bond, and there is a carbon atom at every point where two such straight lines meet. The total number of carbon atoms in oleic acid is 18. Thus, the double bond connects two similar chains of eight carbon atoms, which appear as zigzagged lines in the diagram.
), each single straight line represents a saturated bond, and there is a carbon atom at every point where two such straight lines meet. The total number of carbon atoms in oleic acid is 18. Thus, the double bond connects two similar chains of eight carbon atoms, which appear as zigzagged lines in the diagram.
The trans counterpart of oleic acid, which is produced from it during partial hydrogenation and other high-temperature processes, is called elaidic acid, whose trans carbon–carbon double bond (unsaturated bond) can be seen in the stick diagram of its chemical structure in Elaidic Acid — Compound Summary. In vegetable oils that have not been partially hydrogenated or subjected to high temperatures, all the unsaturated bonds in the fatty acids are double bonds with a cis configuration, as in the case of oleic acid.
How can the difference between the cis and trans configurations be so crucial to our health? Please take a few moments as you are reading this page to do a simple exercise. Sit up straight in your chair, place both of your feet on the floor in front of you, and stretch both of your arms out in front of you. Since our arms are attached to the upper part of our torso and our legs are attached to the lower part, each pair of our limbs (arms or legs) is on the same side (upper or lower). Our bodies thus have a natural cis configuration. The following figure shows the arrangement of our arms and legs alongside the stick diagram of oleic acid, which shows the cis configuration of the hydrogen atoms (H) and chains of atoms attached to the two carbon atoms that are joined by the double bond ( ). The diagram also shows the oxygen atoms (O) and the hydrogen atom (H) in the carboxyl group at one end of the oleic acid molecule. Fatty acids use this group to attach themselves to glycerol when they form triglycerides.
). The diagram also shows the oxygen atoms (O) and the hydrogen atom (H) in the carboxyl group at one end of the oleic acid molecule. Fatty acids use this group to attach themselves to glycerol when they form triglycerides.
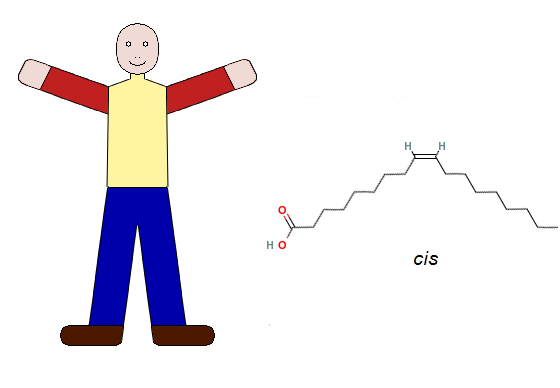
In our cis configuration, we can function normally. Unless, unfortunately, you are handicapped in some way, you can move your arms and hands around and perform all the tasks that you have learned to do on your computer as you sit comfortably in your chair. If you want, you can get up from your chair, move about, and do all the things that you do in the way that you normally do them.
Now imagine that your left arm and left leg exchange places. If this would happen, your left arm would take the place of your left leg, and your left leg would take the place of your left arm. Your two arms would then be diagonally across from one another, as would your two legs. This configuration, in which similar limbs are diagonally across from one another is a trans configuration. The following figure shows the arrangement of the arms and legs in your imaginary body alongside the stick diagram of elaidic acid, which shows the trans configuration of the hydrogen atoms (H) and chains of atoms attached to the two carbon atoms joined by the double bond. As you can see, the rearrangement of the limbs in your imaginary body parallels the rearrangement of the hydrogen atom and the chain of atoms on the right-hand side of the diagram of elaidic acid. This rearrangement is the only structural difference between the natural cis fatty acid and its trans counterpart.
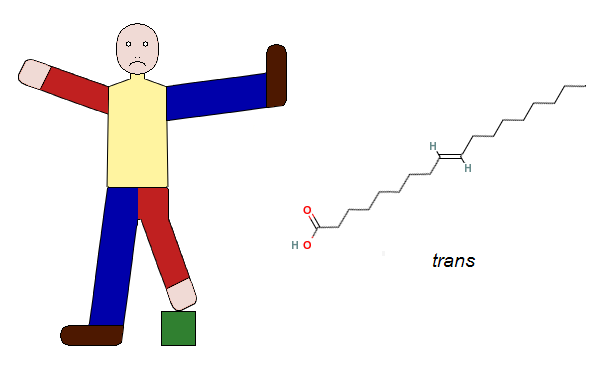
One of the first things that you would probably notice with your imaginary trans body is that you will constantly need to worry about finding a place to rest your left leg. As you go on to think about how you would get up out of your chair and walk, you will quickly realize that a human body with a trans configuration would be extremely clumsy and that any task which requires the participation of both arms or both legs would be far more difficult to perform.
At this point you may be wondering whether the trans configuration can possibly have such drastic effects on the tiny molecules moving about in our body as it would on our body as a whole. It can and it does. However, just as our imaginary trans person would be able to continue to perform many tasks, such as speaking, in the same manner as a person with a cis configuration, the differences in the behavior of trans molecules are manifested in specific processes, while there may be no noticeable difference between the behavior of trans molecules and cis molecules in other processes. Unfortunately, the unique combination of processes in which the differences are manifested in the case of trans fats only compounds the harm that they cause. As you will soon learn, this is a case in which the bad guys have all the luck.
Their Luck Continues and Ours May Be Running Out
The most obvious stroke of good luck that certain trans fats have is the fact that they do not turn rancid. One of the processes that cause oils to become rancid occurs when the double bonds in their unsaturated fatty acids react chemically with oxygen. It appears that the trans double bonds in trans fats cannot easily participate in oxidative rancidity. They thus prevent trans fats from turning rancid and make them attractive to food manufacturers who want to extend the shelf life of their products.
The fats and oils of vegetable origin that we eat are triglycerides. Molecules of triglycerides are formed from one molecule of glycerol and three fatty acid molecules. Before they can be absorbed into our tissues and blood stream, they must be broken down in our digestive system into their fatty acids and glycerol in a process called lipolysis, which is mediated by enzymes called lipases and bile secretions. If we were lucky, the trans bonds would interfere with this process, and trans fats would never enter our blood and tissues. However, triglycerides are broken down at the esteric linkages between the fatty acids and glycerol, far away from the double bonds, whose configuration has little influence on this process. Thus, trans fats are easily digested, and their fatty acids and glycerol are absorbed by cells lining the intestines called enterocytes, in which they are reassembled as triglycerides by enzymes that do not perform a thorough security check on the fatty acid tails to verify that they are natural and do not contain any industrially produced trans double bonds.
Fats are not soluble in our blood and need to be enclosed in a water-soluble (hydrophilic) substance, such as a protein, in order to be transported in our blood. The fat–protein clusters that transport fats in our blood are called lipoproteins, and they are divided into fractions according to their density. Lipoproteins that contain a greater amount of fat (cholesterol and triglycerides) relative to the amount of the surrounding protein are larger and have a lower density, while lipoproteins that contain a smaller amount of fat are smaller and have a higher density. Here the presence of trans double bonds in fat (triglyceride) molecules has a noticeable effect. Triglycerides with trans double bonds (trans fats) cannot be accommodated in the smaller, high-density lipoproteins (HDL). They are consequently transported in our blood in larger, low-density lipoproteins (LDL). This means that any trans fat that we eat should directly increase the amount of LDL in our blood. As luck would have it, high LDL levels are associated with the deposition of fatty substances on the walls of blood vessels and an increased risk of coronary heart disease.
Can I Have Just a Little?
Food products that contain partially hydrogenated oils (trans fats) taste good and stay fresh longer. Most people enjoy eating them and would like to continue doing so. The reactions of individuals to reports about the harm that trans fats may cause vary from not making any change in choosing the foods that they eat to completely stopping to consume any product that contains any trans fats and joining campaigns to ban them. If you continue eating products that contain trans fats, you are, in effect, deciding for yourself how much of a risk you are willing to take.

